Blue-blooded crabs at heart of pharma dispute on drug testing
06 August, 2019
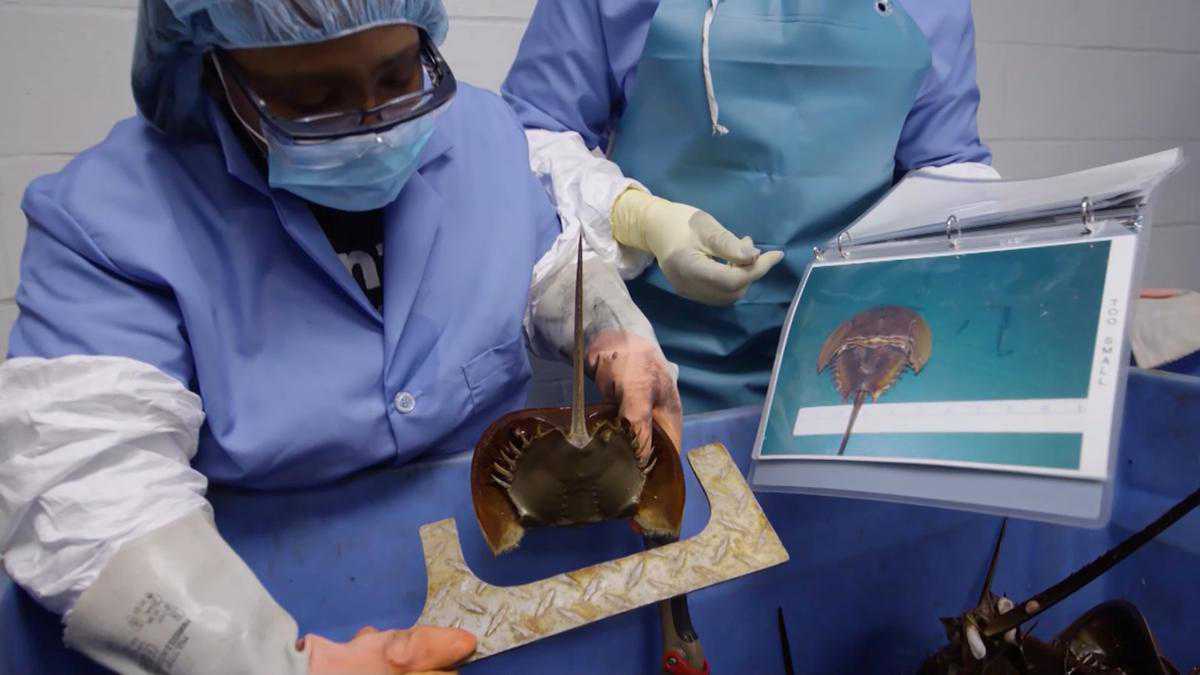
If you've received a vaccination, hip replacement or chemotherapy without suffering toxic shock, chances are you've benefited from a vast bloodletting of horseshoe crabs.
Every year, fishermen net hundreds of thousands of the creatures off the U.S. East Coast and in Asia before their prized milky-blue blood is drained for use in medical safety tests.
Swiss biotech Lonza and U.S.-based Charles River Laboratories are the biggest suppliers of crab blood-based endotoxin tests, which detect bacterial contamination in intravenous drugs and medical implants.
They are now at odds over the future of this testing, as Lonza urges adoption of a synthetic alternative called recombinant Factor C (rFC), amid pressure from wildlife campaigners and worries about supply reliability.
Meanwhile Charles River, which is still studying rFC, argues moving too quickly could compromise patient safety.
There's much at stake - for both companies, the industry and, of course, horseshoe crabs, a 450-million-year-old species that's more closely related to scorpions than crustaceans.
Endotoxin tests number 70 million annually and are rising. If rFC gains traction, it could re-shuffle winners and losers in the global market for such testing that is seen growing about 13% a year to close to $700 million in annual revenue by 2021 and $1 billion by 2024, according to market researchers.
For Lonza, whose internal testing growth estimates are more modest, the risk is that the synthetic method won't catch on, hurting efforts to capitalize on a $460 million investment it made in 2006 when it bought the technology along with a peer's biological businesses.
Conversely, Charles River risks becoming a laggard if the technique wins wide acceptance.
PHARMACOPOEIA PUSH
The leading U.S. pharmaceuticals standards organization, U.S. Pharmacopoeia (USP), which publishes a compendium of drug information, told Reuters it was pressing ahead to recognize synthetic tests as comparable to crab blood-based ones.
"There is a consensus on moving forward and USP is fully committed," said Fouad Atouf, USP's Science-Global Biologics head.
"Making sure all the stakeholders are on board and all the data has been collected is what's happening right now," he said, with proposed revisions recognizing rFC due around September for public consultation.
That coincides with a push by USP's European counterpart in Strasbourg, to recognize rFC. Japan is also working on the issue.
France's Biomerieux and Germany's privately held Haemochrom Diagnostica, which have versions of rFC, are also eager to see pharmacopoeias act.
Lonza said its rFC product, PyroGene, now accounts for less than 5 percent of its endotoxin test sales but sees fortunes changing as global pharmacopoeias act.
"We would hope to see around 10% by 2021," Lonza said.
It has won a big customer for PyroGene: Eli Lilly's migraine medicine Emgality last year became the first rFC-tested drug to win U.S. regulatory approval.
Roche and Bayer told Reuters they were also considering rFC.
Charles River insists additional scrutiny is necessary before rFC technology wins equal standing.
Companies pushing rFC must prove it can detect endotoxins in the real world, not just the manufactured strains used so far in studying the synthetic agent, said John Dubczak, Charles River's microbial solutions manager.
"A move by the pharmaceutical industry towards current rFC methods is premature," said Dubczak, adding more data is needed.
Lonza points to analyses by multiple drugmakers demonstrating rFC was comparable to the crab-blood tests in ensuring drugs were contaminant-free.
To be sure, Lonza has no plans to stop bleeding crabs, since many existing medical products will continue to be tested with traditional methods, it said.
The industry predicts the global crab blood-based test market will also expand in the next few years.
But Lonza is counting on rFC emerging as a new pillar of its testing business, contributing to its goal of more than 7 billion Swiss francs ($7.1 billion) in group annual revenue by 2022, from 5.5 billion francs.
Horseshoe crabs' copper-rich blood has helped them survive since before the dinosaurs in brackish coastal waters by clotting around endotoxins, the potentially dangerous molecules found in cell walls of bacteria like E. coli.
Scientists harnessed nature's ingenuity, using crab blood to make so-called amebocyte lysate endotoxin tests which, by the 1970s, began displacing tests on rabbits that were injected with medicine then monitored for fever.
However, concerns have since escalated over wildlife conservation and the supply chain's reliability.
In Asia, where horseshoe crabs are also eaten by humans, the tri-spine species is listed by the International Union for Conservation of Nature as at risk of extinction. Two other species are also imperiled.
Glenn Gauvry, director of the Delaware-based Ecological Research and Development Group which focuses on horseshoe crab conservation, said Asian crabs destined for dinner tables were first "rented" to biomedical companies for bleeding.
Gauvry contends the biomedical industry is perpetuating consumption of crabs in Asia and contributing to their decline.
Lonza does not harvest Asian crab blood.
Charles River, which does, said it followed local rules.
"Horseshoe crab blood is collected only to a point where the crab naturally stops bleeding," Dubczak said, adding its Asian suppliers and local governments were responsible for ensuring crabs were released into "waters of appropriate salinity".
Studies paint a mixed picture of the blood harvest's effects on U.S. East Coast horseshoe crabs, hundreds of thousands of which are also killed annually for bait or caught incidentally by commercial fishermen hunting other marine species.
Those caught by Lonza, Charles River and Associates of Cape Cod, owned by Japan's Seikagaku Corp, are drained of about a third of their blood, then returned alive to the sea.
An Atlantic States Marine Fisheries Commission report from May indicates current harvest levels "appear to be sustainable".
Additionally, a recent U.S. Geological Survey study found bled crabs' survival rates may exceed those of non-bled crabs in New Jersey's Delaware Bay.
However, previous studies found anywhere between 4% to 30% of bled crabs may die prematurely, prompting pressure on the biomedical industry, largely out of concern for shorebird populations that rely on crab eggs.
Advocates including the Audubon Society became increasingly vocal when the rufa red knot, which eats crab eggs on stopovers at Delaware Bay, was listed as threatened under the U.S. Endangered Species Act in 2014.
"That was the tipping point," said Jay Bolden, an Eli Lilly biologist whose hobby as a birdwatcher spurred him to champion his company's switch to rFC that he believes will ensure supply-chain reliability.
"If it got really serious, and they said nobody could touch the horseshoe crabs because they have to rebuild the red knot population, that was going to be significant."
Lilly has shifted three of eight global drug manufacturing sites to rFC testing and is more than halfway to its goal of 90 percent rFC testing by 2020, he added.
"Until the global pharmacopoeias revise the test method to recognize rFC as on par with crab blood-based tests, we can't just make the change for medicines that are already on the market," Bolden said. "When that happens, then we can change over that final 10 percent."
Source:
