Hope during COVID-19: Russian vaccine holds guarantee and other findings
13 September, 2020
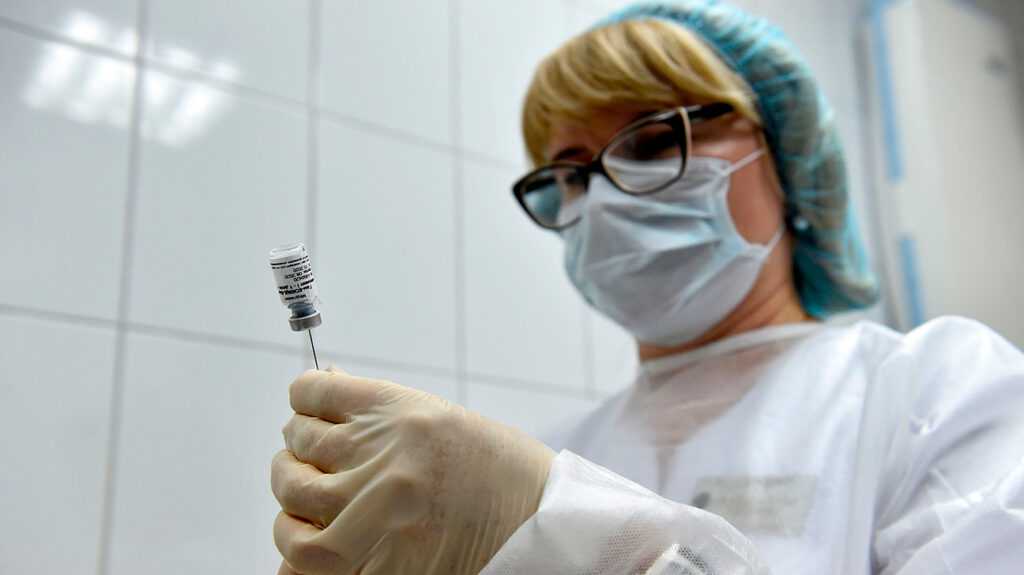
While the pandemic continues to be ongoing, we continue steadily to round up the scientific tests that offer encouraging results to help us envisage an end to the current crisis.
Firstly, scientists at the Gamaleya Research Institute of Epidemiology and Microbiology, in Moscow, devised a vaccine that elicits a robust immune response, without serious side effects in humans.
The previous few weeks also saw the expansion of a considerably faster COVID-19 ensure that you an intranasal vaccine that was effective in mice.
Finally, a meta-analysis helps settle the matter of whether blood circulation pressure drugs make COVID-19 better or worse, while an arthritis drug may reduce serious illness.
Russian vaccine secure, triggers immune response
The Russian vaccine, known as Sputnik V, runs on the altered version of an adenovirus that causes the common chilly. Denis Logunov, the study’s first writer and mind of the study lab at Gamaleya, clarifies how it works.
“When adenovirus vaccines enter people’s cells, they deliver the SARS-CoV-2 spike protein genetic code, which in turn causes cells to create the spike protein. This helps teach the immune system to recognize and strike the SARS-CoV-2 virus,” Logunov says.
The researchers assessed the vaccine in a small phase 1 and 2 trial that lasted 42 days and included 38 healthy participants aged 18-60.
At this stage in the study, the scientists wished to assess the vaccine’s safe practices and its capability to elicit an immune response, instead of whether it could prevent infections with the brand new coronavirus.
The results showed a solid immune response in the participants. They created antibodies and more advanced immune responses, very similar to T cell reactions. T cells happen to be white immune cells - a few of which, called killer T cells, will get and eliminate infected cells.
The Russian vaccine was also found to be safe and didn't elicit any serious side effects. Next, the workforce plans to enter phase 3 of the trial, that may involve 40,000 individuals.
Repurposing an arthritis drug
Japanese-based mostly scientists from Osaka University and Osaka Habikino Medical Center believe they could repurpose an arthritis drug to treat severe COVID-19.
The team began their research by concentrating on cytokines, proteins secreted by immune cells, with key immunomodulatory functions. They aimed to comprehend their function in cytokine storms - the immune system phenomenon that may result in fatal outcomes in COVID-19.
With this thought, the scientists studied the cytokine profiles of 91 clients with cytokine storms because of “bacterial sepsis, acute respiratory distress syndrome, or burns.”
After concentrating on various interleukins - a kind of cytokine - the scientists found a growth in interleukin-6 (IL-6) early on in the disease approach, which piqued their interest.
In turn, this upsurge in IL-6 triggered a growth in a protein called PA-1, which contributes to blood clotting.
Thus, the scientists gave individuals injections of the antibody-based medicine tocilizumab (Actemra), which blocks IL-6 signaling.
When people with severe COVID-19 received tocilizumab, their levels of the bloodstream clotting PA-1 protein reduced. Also, the medication eased symptoms and better significant illness for these patients.
Intranasal vaccine works on mice
Researchers from Washington University School of Medicine found in St. Louis (WUSTL) possess devised a new intranasal vaccine, tested in mice with COVID-19.
Like the Russian vaccine, WUSTL’s counterpart also runs on the modified adenovirus inserted with the coronavirus’ spike protein.
In mice, one vaccine dose shielded against infection with SARS-CoV-2.
Talking with MNT about their findings, senior examine author Prof. Michael S. Diamond discussed that the intranasal vaccine wouldn't normally use a live type of the virus. This made it safer than different vaccines administered through the nasal area, like the regular influenza vaccine.
“In these mouse models, the vaccine is highly protective. We’re pumped up about beginning another round of analyses and in the end testing it in people to see if we are able to induce the type of protective immunity that people think can not only prevent illness but also curb pandemic transmitting of the virus,” said Prof. Diamond.
“Any vaccine can cause side effects,” he continued. “On the other hand, because the vaccine will not replicate, it might not cause unanticipated an infection just how a ‘live’ vaccine could - in this way, it could be safer.”
“We will soon begin a study to check this intranasal vaccine in nonhuman primates with an idea to move into human being clinical trials as quickly as we are able to.”
- Prof. Michael S. Diamond
Source: www.medicalnewstoday.com
