Important European nations suspend use of AstraZeneca vaccine
16 March, 2021
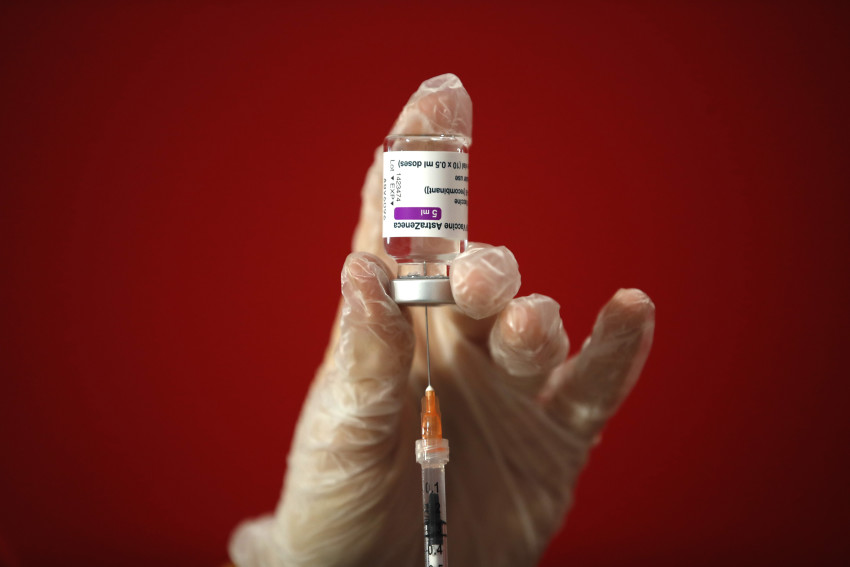
A growing number of Europe - including Germany, France, Italy and Spain - suspended utilization of AstraZeneca’s COVID-19 vaccine Monday over studies of dangerous blood clots in a few recipients, although company and overseas regulators say there is absolutely no evidence the shot is at fault.
AstraZeneca's formula is merely among three vaccines used on the continent. But the escalating concern is definitely another setback for the European Union's vaccination get, which has been suffering from shortages and additional hurdles and is certainly lagging very well behind the promotions in Britain and the U.S.
The EU's medicine regulatory agency called a meeting for Thursday to examine experts' findings on the AstraZeneca shot and make a decision whether action has to be taken.
The furor comes as a lot of Europe is tightening restrictions on schools and businesses amid surging cases of COVID-19.
Germany's wellbeing minister said your choice to suspend AstraZeneca pictures was taken on the suggestions of the country's vaccine regulator, the Paul Ehrlich Institute, which called for further investigation into seven cases of clots found in the brains of men and women who was simply vaccinated.
“Today’s decision is a purely precautionary measure,” Jens Spahn said.
French President Emmanuel Macron said his region will likewise suspend shots until at least Tuesday afternoon. Italy’s drug regulator as well announced a short-term ban, as does Spain, Portugal and Slovenia.
Other countries which may have taken comparable action in the last couple of days include Denmark, the first ever to do so, and also Ireland, Thailand, the Netherlands, Norway, Iceland, Congo and Bulgaria. Canada and Britain will be position by the vaccine for the present time.
In the coming weeks, AstraZeneca is expected to apply for U.S. authorization of its vaccine. The U.S. now depends on Pfizer's, Moderna's and Johnson & Johnson's vaccines.
AstraZeneca said there were 37 reports of bloodstream clots out greater than 17 million persons vaccinated in the 27-country EU and Britain. The drugmaker explained there is no facts the vaccine carries an increased risk of clots.
In fact, it explained the incidence of clots is a lot lower than would be likely to occur naturally in an over-all population of the size and is similar to that of additional licensed COVID-19 vaccines.
The World Health Company and the EU's European Medicines Agency have also said that the info does not suggest the vaccine caused the clots and that people should continue being immunized.
“Many thousands of folks develop blood clots annually in the EU for distinct reasons,” the European Medicines Agency said. The incidence in vaccinated persons "seems never to be greater than that viewed in the overall population.”
The agency said that while the investigation is certainly going on, “the benefits associated with the AstraZeneca vaccine in protecting against COVID-19, using its associated risk of hospitalization and loss of life, outweigh the risks of unwanted effects.”
Blood clots can travel through the body and cause center episodes, strokes and deadly blockages in the lungs. AstraZeneca reported 15 conditions of deep vein thrombosis, or a kind of clot that often develops in the legs, and 22 cases of pulmonary embolisms, or clots in the lungs.
The AstraZeneca shot has turned into a key tool in European countries' efforts to improve their sluggish vaccine rollouts. Additionally it is pillar of a U.N.-backed project referred to as COVAX that aims to supply COVID-19 vaccines to poorer countries. That plan continues unaffected by the European suspension.
Pfizer's and Moderna's vaccines are also used on the European continent, and J&J's one-shot vaccine features been authorized however, not yet delivered.
Dr. Michael Head, a senior analysis fellow in global wellness at the University of Southampton in England, explained there is absolutely no data yet to justify suspending the AstraZeneca vaccine and known as your choice “baffling."
“Halting a good vaccine rollout throughout a pandemic has implications,” Head said. “This effects in delays in guarding people, and the prospect of increased vaccine hesitancy, consequently of people who've seen the news and understandably become concerned."
Spahn, the German health minister, said of the decision to avoid using the AstraZeneca vaccine: “The main thing for self-confidence is transparency.” He said both primary and second doses will be suspended.
Germany has received slightly above 3 million doses of the AstraZeneca vaccine, and about 50 % of those have up to now been administered, compared with nearly 7 million of the Pfizer shot and about 285,000 from Moderna.
German authorities have encouraged anyone who feels increasingly ill a lot more than four days after obtaining the shot - for example, with persistent headaches or perhaps dot-shaped bruises - to get medical attention.
The head of the Spanish Medicines Agency, Maria Jesús Lamas, said Spain detected its first case of clots on Saturday. She explained the ban was “not a fairly easy decision” since it further slows the nation's vaccination campaign, but it was the “most prudent” approach.
Almost 940,000 people in Spain have received the AstraZeneca shot.
Some Europe, meanwhile, have begun reimposing restrictions in a bid to beat back a resurgence in infections, most of them from variants of the initial virus.
In Italy, 80% of children nationwide couldn’t attend classes after stricter rules in extra regions took effect on Mon. In Poland, bolstered constraints were applied to two more regions, incorporating Warsaw. Paris could get into lockdown in a matter of times because intensive care systems are receiving swamped with COVID-19 patients.
And calls are growing in Germany to “ draw the emergency brake” in regions where instances are rising.
Source: japantoday.com
