India hails 'life saving' COVID-19 vaccine rollout
18 January, 2021
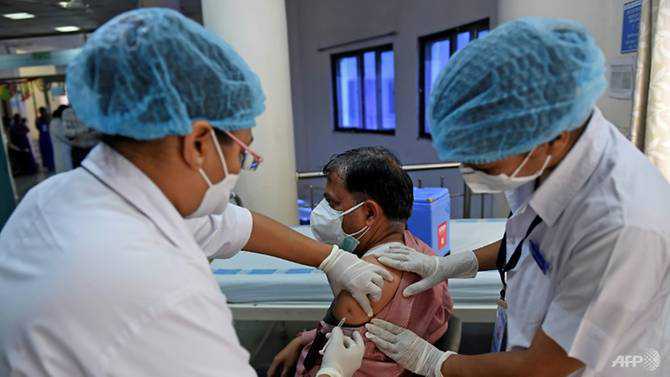
India's COVID-19 vaccination drive had an effective start with more than 190,000 people acquiring their initial jabs and no person hospitalised for major unwanted effects, medical ministry said, but reports emerged about problems over the homegrown vaccine.
Authorities have got given emergency-use approval for just two vaccines - Oxford-AstraZeneca and the homegrown Covaxin, which includes yet to complete it has the Period 3 trials - and strategies to immunise around 300 million people in the region of just one 1.3 billion by July.
Frontline staff such as hospital staff, people over 50 and the ones deemed to be at high risk thanks to pre-existing medical ailments are about the shortlist to receive the vaccines.
"We've got encouraging and satisfactory responses results on the 1st day," Well being Minister Harsh Vardhan advised his status counterparts on Saturday (Jan 16).
"This vaccine will indeed be considered a 'Sanjeevani' (life saver)" in the fight the virus, he added.
Medical ministry said "no case of post-vaccination hospitalisation" have been reported, although native media said a security guard at the country's top-ranked public medical center, the All India Institute of Medical Sciences (AIIMS) in Delhi, had developed an allergic reaction soon after getting his shot.
A doctors' representative human body at the Ram Manohar Lohia Medical center in New Delhi wrote a letter requesting the Oxford-AstraZeneca Covishield vaccine to end up being supplied rather than Covaxin to allay any fears.
"The residents will be somewhat apprehensive about having less complete trial in the event of Covaxin and may not take part in huge numbers therefore defeating the purpose of vaccination," said the letter addressing the hospital's medical superintendent, seen by AFP.
"We demand you to vaccinate us with Covishield, which includes completed all stages of trial before its rollout."
Pathologist Arvind Ahuja told AFP in the hospital on Saturday that he shared some of the concerns.
"I hope when the info comes out, it really is good. Ideally, they must have waited for just one month at least as then we'd have known better about its efficacy," the 45-year-old said.
Vaccine hesitancy offers emerged as a major concern, with a recently available survey of 18,000 persons across India finding that 69 per cent were in no hurry to acquire a shot.
Leading scientists and doctors have named on authorities release a efficacy data about Covaxin to improve confidence about the vaccine.
Covaxin recipients on Saturday had to signal a good consent form that stated its "clinical efficacy ... is definitely however to be established".
Officials had hoped to inoculate 300,000 persons on Saturday but said glitches with a great software used to coordinate and screen the process meant not absolutely all probable recipients were alerted.
India gets the world's second-largest known caseload with an increase of than 10.5 million coronavirus attacks and over 152,000 deaths up to now.
Source:
