Pharma Firm Loses License for Gene Therapy Drug
29 May, 2019
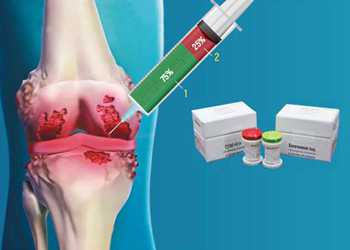
Pharma firm Kolon Life Sciences on Tuesday lost its license for an osteoarthritis gene therapy drug called Invossa for falsely reporting a key ingredient.
Invossa was Korea's first gene therapy drug for patients with the degenerative joint ailment. But health authorities revoked the license because Kolon did not use cartilage cells to manufacture the drug, as authorized on application, but kidney cells.
Kolon claims the misreporting was unintentional.
The development has been disastrous for the company. Korea Exchange is considering de-listing Kolon Tissue Gene, the subsidiary that manufactured Invossa after halting trading in the company's shares. Kolon Life Sciences shares were also suspended, but trading resumed Wednesday.
Some 244 patients who received Invossa shots have filed a class action against both companies in the Seoul Central District Court. More plaintiffs are expected to join since a total of 3,707 injections of the drug were administered to patients.
The plaintiffs are demanding W2.5 billion for pain and suffering as well as spending on the drug, which cost a staggering W7 million per shot (US$1=W1,190).
"Many patients, usually older ones, already felt sorry for their children who paid for the shots because they knew how much it costs, and now the children feel guilty for putting their parents in danger," said a spokesman for Oh Kims Law and Company, which represents the plaintiffs.
TAG(s):
