Japan's success in curbing COVID-19 instances now hampers seek out cures
24 June, 2020
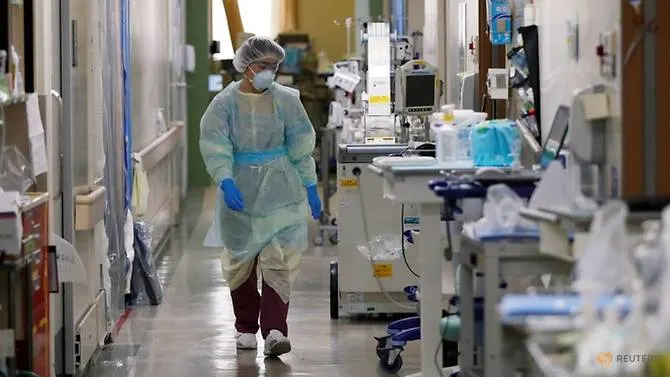
As nations race to develop treatments and vaccines for COVID-19, Japan has become a victim of its success as slowing latest infections has led to a shortage of sufferers to enroll in clinical trials.
Clinical trials are underway for more than a dozen potential vaccines, including at least six in China, but Japan's first human trials are anticipated to start the following month.
In development of treatments, Russia and India approved Fujifilm Holdings Corp's Avigan as a COVID-19 therapy, but Japan, whose Primary Minister Shinzo Abe has touted the drug's potential and hoped to approve it in-may, won't see a decision until at least July.
"As a result of decreasing number of coronavirus infections, we believe it will require time before clinical study is completed," stated Tetsuya Nakamura, who is owning a trial of Avigan at Gunma University Medical center in central Japan.
"It's a pity that Avigan provides been approved overseas however, not in Japan."
Japan has fared much better than most developed nations found in tackling the disease that has killed a lot more than 470,000 worldwide. While the epidemic drove Japan's medical program to the brink of collapse lately, serious cases now number about 60 nationwide.
Some 54 COVID-19 related clinical trials have already been launched in Japan, but the majority are still in the individual recruitment stage, according to trials' tracking data.
Interest found in Avigan, known generically while favipiravir, soared found in March after a good Chinese official said it appeared to help patients recover from COVID-19. It really is now the main topic of at least 25 clinical trials all over the world.
The regulatory delay on Avigan is partly because of the fact that the studies must have been completed in multiple countries simultaneously, said Dr. Nakamura. But such analyses are "enormously expensive."
Fujifilm said it really is attempting to complete the clinical trials "immediately."
Japanese biotech strong Healios KK said in April it intended to add COVID-19 affected individuals to its experimental lung therapy trial but hasn't enlisted any up to now.
"We had been careful to size the cohort in light of the reduced number of sufferers in Japan, and so are only seeking to enroll around five sufferers," CFO Richard Kincaid stated.
With a dearth of domestic patients, Japan may need to rely more on overseas data and leads to assist in regulatory approvals. That practice is usually common "if the grade of data is known as to be good enough," according to wellness ministry official Yasuyuki Sahara.
Sahara did not touch upon the Russia or perhaps Indian approvals of Avigan and whether data from those countries could be used in Japan. The Pharmaceuticals and Medical Products Agency, Japan's primary medication regulator, did not immediately react to a obtain comment.
Source:
TAG(s):
